Carbon management makes use of many technologies, which include carbon capture, utilization, and storage (CCUS). The PCOR Partnership region has significant potential to reduce or offset anthropogenic CO2 emissions to the atmosphere with natural storage options at the surface and deep underground.
There are two major types of CO2 storage: geologic and terrestrial.

Terrestrial, or biological, CO2 storage uses land management practices to maximize the amount of carbon stored in plant roots and organic matter in the soil. Two major strategies are protecting ecosystems that store carbon in order to maintain or increase their carbon stores and actively managing soils and plants to increase carbon storage beyond the current conditions through natural processes such as photosynthesis. No-till farming, wetland management, rangeland management, and reforestation are examples of terrestrial sequestration practices that are already in use.

Geologic CO2 storage permanently stores CO2 deep underground and is one component of CCUS. By capturing CO2 at the source instead of releasing it to the atmosphere, CCUS can greatly reduce CO2 emissions from large point sources such as coal- and gas-fired power plants, natural gas-processing facilities, ethanol plants, and other industrial facilities.
The steps of CCUS are the capture of CO2 by separation from other gases, compression to a liquid or dense fluid state, transport to a geologic storage site, and injection into deep geologic formations. This ensures permanent storage, isolating CO2 from the atmosphere.
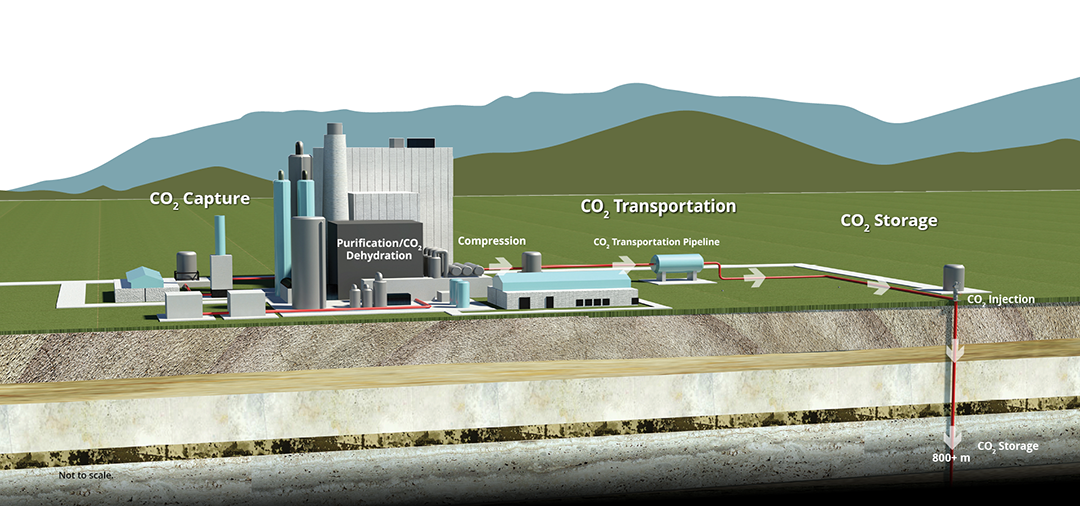
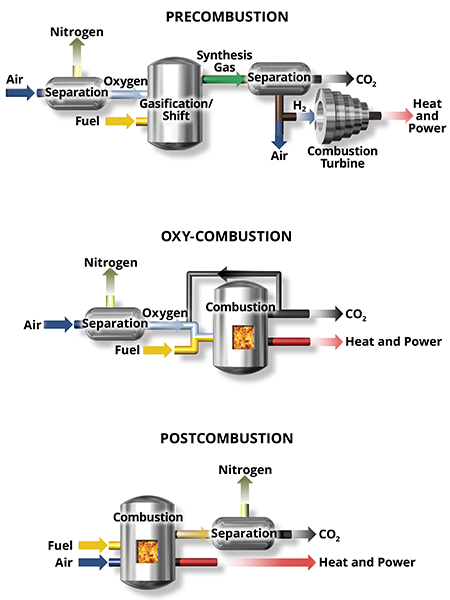
Capture is the separation of CO2 from a gas stream to prevent atmospheric release. Capture can be performed before, during, or after the combustion process.
Precombustion technologies consist of capturing CO2 in conjunction with either gasification or methane reforming to produce hydrogen for use in a turbine.
Capture during combustion is possible when the oxygen source is pure oxygen rather than air (called oxy-combustion or oxy-fuel combustion).
The majority of capture technologies focus on separating low-concentration CO2 from the exhaust gas stream after combustion takes place; this is called postcombustion capture. This technique is the most common for retrofitting existing systems.
CO2 makes up a relatively small fraction of the exhaust gas mixture, but at a utility or industrial scale, the amount of CO2 can be quite large. Because it involves complex chemistry and specialized equipment, capturing CO2 is expensive.

Captured CO2 must be dehydrated and compressed into a supercritical or liquidlike state for either truck
transport or piping to the storage site. CO2 must be compressed to at least 1200 to 1500 psi for transport
in a pipeline to ensure that it remains in a dense state. Because compression is energy-intensive, improved compression
methods are under development.
Following capture and compression, CO2 is transported to a storage site. Given the quantities of CO2
that are likely to be captured from industrial sources, pipelines appear to be the most likely mode for transporting the
captured gas to geologic storage sites.
Geologic storage involves injecting captured anthropogenic CO2 into deep underground geologic formations. Permanent storage can occur in either dedicated storage projects or through enhanced oil recovery (EOR) efforts.
When CCUS projects are designed for dedicated storage, CO2 is injected into a carefully selected storage formation to permanently store it underground. When CCUS occurs as part of an EOR project, the CO2 is injected into an existing oil-bearing reservoir to increase oil production. A portion of the CO2 will be brought back to the surface with the oil where it is then separated and reinjected into the same reservoir.
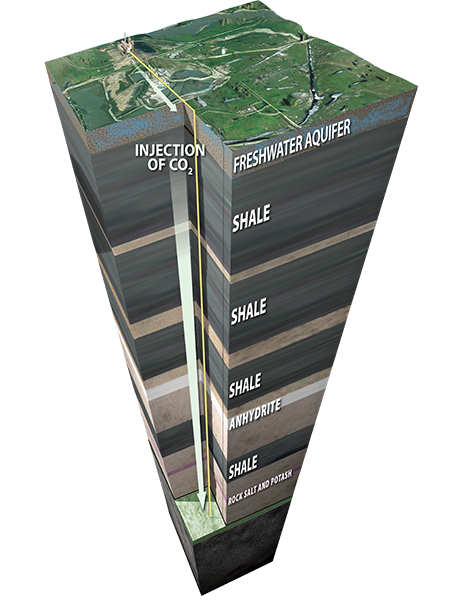
Picking the right geologic location is critical to the safe, permanent storage of CO2. Successful storage requires that the CO2
stays in the injection layer, also known as the storage layer. Important factors in identifying appropriate storage reservoirs include:
Capable of storing large quantities of CO2 permanently.
Thick, laterally continuous seals or cap rocks that prevent upward migration of CO2.
No geologic faults in the surrounding rocks.
Ample barriers between the storage zone and sources of drinking water (typically deeper than 3000 feet [~800 meters]).
Rock content compatible with CO2 injection and storage.
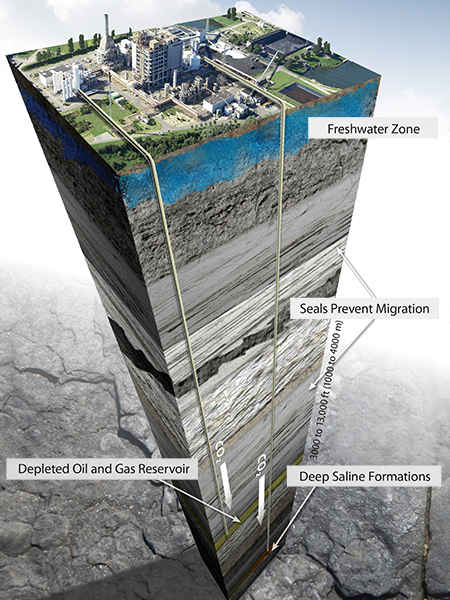
Many suitable areas across the globe have the capacity to hold CO2 emissions safely and securely deep underground. These formations exist in sedimentary basins and include oil and gas fields and saline formations. What these formations have in common is a porous and permeable storage layer sealed by impermeable cap rocks.
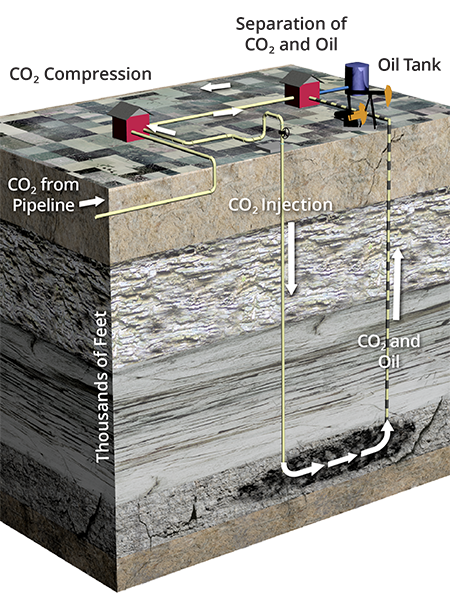
Oil reservoirs have many characteristics that make them excellent target locations to store CO2. The geologic conditions that trap hydrocarbons are the same as needed for permanent CO2 storage. Oil fields have many characteristics that make them excellent target locations to store CO2. The geologic conditions that are conducive to hydrocarbon accumulation are also the conditions that are conducive to CO2 storage.
The three requirements for trapping and accumulating hydrocarbons are a hydrocarbon source, a reservoir layer with connected pore space to allow flow and storage, and impermeable seals—cap rocks above the reservoir that prevent escape.
A single oil field can have multiple zones of accumulation, i.e., oil reservoirs and more than one depth.
Like oil, supercritical CO2 is lighter than the ancient ocean water in the oil reservoir. Injection pushes the CO2 outward from the well to flow upward through the tiny paths between rock grains until the CO2 reaches an impermeable seal. In an EOR operation, the CO2 will mix with any oil it encounters, swelling and mobilizing more oil for production. In the long term, the CO2 will dissolve into the formation fluids and eventually mineralize, becoming part of the formation rock.
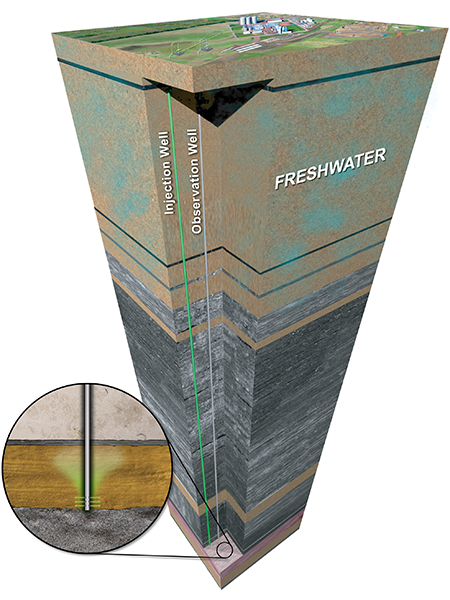
Sedimentary basins are relatively large areas of Earth’s surface that have subsided over long periods of
geologic time, allowing for the accumulation of sediments that eventually lithified into rock. Areas where
the accumulation of sediments is thick enough (>800 meters) may have an arrangement of rock layers suitable
for CO2 storage. Many sedimentary basins are home to hydrocarbon accumulations that are being
tapped in oil and gas fields worldwide.
Most rock layers in sedimentary basins do not have a hydrocarbon source but are often saturated with brine.
These layers are called saline formations and are widely distributed throughout North America and the rest
of the world. Their geologic characteristics, global spread, and proximity to many large-scale CO2
sources mean deep saline formations account for most of the world’s geologic storage resource. They also
provide an ideal storage option for facilities not able to take advantage of economic CO2 EOR opportunities.
Saline formations suitable for CO2 storage comprise sandstone, limestone, dolomite, or some mix of the
three. Many of these formations are ideally situated to provide great potential for CO2 storage and are
also overlain by thick and regionally extensive cap rocks, typically shales and salts. These cap rocks
function as seals to help ensure that the injected CO2 remains in place permanently.